When you pick up a generic pill at the pharmacy, you expect it to work just like the brand-name version. But how does the FDA make sure it actually does? The answer lies in bioequivalence studies - the science behind proving that a generic drug behaves the same way in your body as the original.
Why Bioequivalence Matters
Generic drugs save patients and the healthcare system billions each year. In the U.S., they make up 90% of all prescriptions but cost only 23% of what brand-name drugs do. That’s a huge win - but only if they’re truly interchangeable. The FDA doesn’t just accept claims. It demands proof.Before a generic drug can be approved, manufacturers must show two things: pharmaceutical equivalence and bioequivalence. Pharmaceutical equivalence means the generic has the same active ingredient, strength, dosage form, and route of administration as the brand-name drug. That’s the easy part. Bioequivalence is where the real testing begins.
Bioequivalence means the generic drug delivers the same amount of medicine into your bloodstream at the same rate as the original. If it doesn’t, you could get too little - and the drug won’t work - or too much - and risk side effects. For drugs with a narrow therapeutic index, like warfarin or levothyroxine, even small differences can be dangerous.
How the FDA Tests Bioequivalence
The gold standard is a clinical study in healthy volunteers. Typically, 24 to 36 people take both the generic and the brand-name drug in separate sessions, under strict conditions. Usually, they fast overnight before taking the drug to avoid food interfering with absorption. Blood samples are drawn over several hours to track how the drug moves through the body.The FDA looks at two key numbers: AUC and Cmax.
- AUC (Area Under the Curve) measures total drug exposure over time - how much medicine your body absorbs overall.
- Cmax (Maximum Concentration) shows how fast the drug reaches its peak level in your blood.
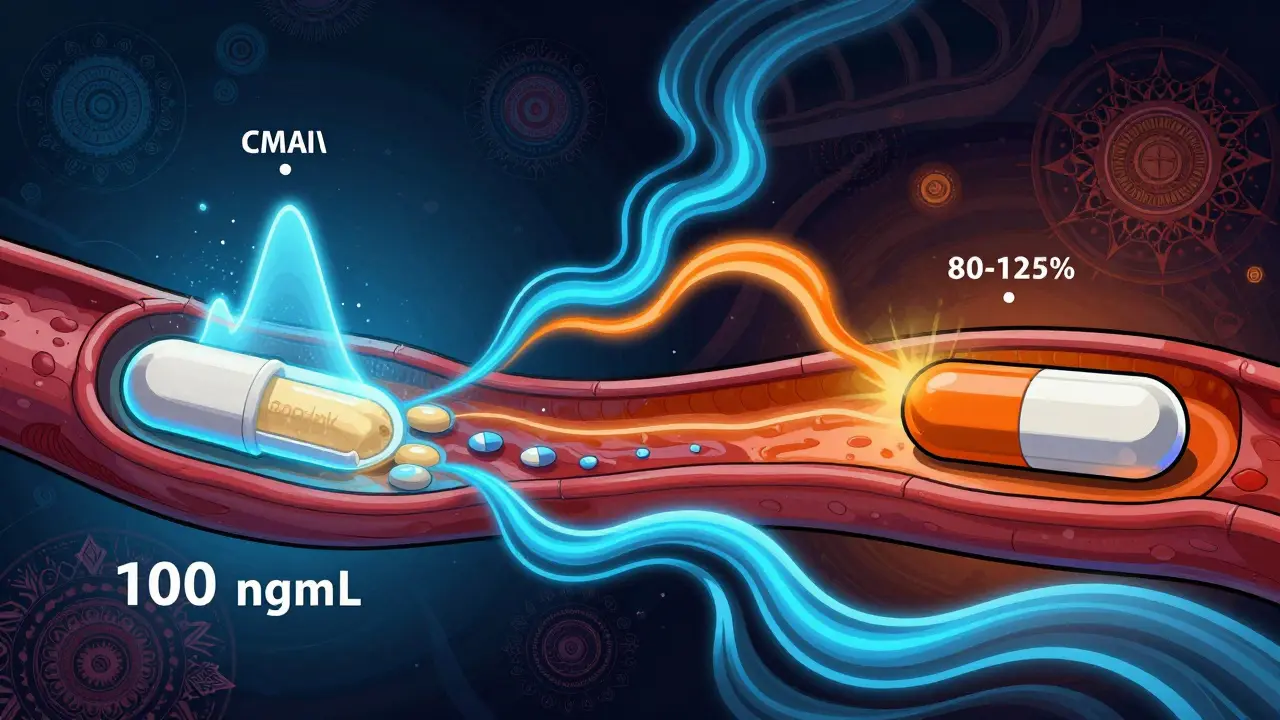
For the generic to pass, the 90% confidence interval for the ratio of the generic’s AUC and Cmax compared to the brand must fall between 80% and 125%. This is known as the 80/125 rule. It’s not a guess - it’s based on decades of data showing that if two drugs meet this standard, they’ll produce the same therapeutic effect in most patients.
For example, if the brand-name drug reaches a Cmax of 100 ng/mL, the generic must reach between 80 and 125 ng/mL. Same for AUC. This range accounts for normal biological variation between people. It’s strict, but it works.
When the FDA Allows Biowaivers
Not every drug needs a full clinical study. The FDA allows biowaivers - exceptions - for certain products where absorption isn’t a concern.For example:
- Topical creams or ointments meant to work on the skin (not absorbed into the blood) can be approved using in vitro tests that measure how the drug releases from the product.
- Eye drops or ear drops with the same ingredients and concentration as an approved product don’t need human trials.
- Some oral solutions and inhaled anesthetics also qualify.
To qualify for a biowaiver, the generic must match the brand exactly in three areas - known as the Q1-Q2-Q3 rule:
- Q1: Same active and inactive ingredients.
- Q2: Same dosage form and strength.
- Q3: Same pH, solubility, and other physical properties.
If all three match, the FDA assumes the drug will behave the same way. This saves time and money - and helps get affordable generics to market faster.
Special Cases: Narrow Therapeutic Index Drugs
Some drugs have very little room for error. Warfarin, for example, is a blood thinner. Too little, and a patient risks a clot. Too much, and they could bleed internally. Levothyroxine, used for thyroid conditions, needs precise dosing to avoid heart problems or weight swings.For these drugs, the FDA tightened the rules. Instead of 80-125%, the acceptable range for AUC and Cmax is now 90-111%. That’s a much smaller window. It means manufacturers must be even more precise in their formulation and testing.
These stricter standards are based on real-world outcomes. Studies show that switching between brands and generics for NTIDs can lead to more hospital visits if bioequivalence isn’t tightly controlled.
What Can Go Wrong - And How Manufacturers Avoid It
Getting bioequivalence approval isn’t easy. In 2022, only 43% of generic drug applications passed on the first try. The most common reasons for rejection?- Study design flaws - like too few participants or poorly timed blood draws.
- Inaccurate lab methods - if the lab can’t measure drug levels precisely, the data is useless.
- Missing documentation - the FDA requires every step to be recorded, from how the drug was stored to how samples were handled.
- Ignoring product-specific guidances.
Companies that follow the FDA’s product-specific guidances (PSGs) have a much better shot. There are over 2,100 of these documents, each tailored to a specific drug. They tell manufacturers exactly what tests to run, what conditions to use, and what data to submit. Following them bumps first-cycle approval rates from 29% to 68%.
One major pitfall? Assuming a bioequivalence study that worked for one drug will work for another. Each drug behaves differently. A study design that works for an antibiotic might fail for a cholesterol drug. That’s why PSGs exist - to prevent one-size-fits-all mistakes.

New Tools and Future Changes
The FDA isn’t stuck in the past. It’s using new science to make testing smarter.For complex drugs - like inhalers, topical gels, or injectables - traditional blood tests aren’t always enough. So the FDA now accepts:
- Physiologically Based Pharmacokinetic (PBPK) modeling - computer simulations that predict how a drug moves through the body based on its chemistry and human physiology.
- Advanced in vitro tests - lab-based methods that mimic how a drug behaves in the body without needing human volunteers.
- Scaled average bioequivalence (SABE) - a statistical method for drugs with high variability, where the 80/125 rule is too strict.
These tools are especially helpful for generic versions of complex products like inhalers or long-acting injectables. They cut development time and reduce costs without sacrificing safety.
The FDA is also pushing for more U.S.-based testing. Its Domestic Generic Drug Manufacturing Pilot Program gives faster review to generics made with American-sourced ingredients and tested in U.S. labs. This helps reduce supply chain risks and supports domestic production.
What This Means for Patients
You don’t need to understand AUC or Cmax to benefit from bioequivalence studies. But knowing they exist should give you confidence.Every generic you take has gone through rigorous testing. The FDA doesn’t approve a drug just because it looks the same or costs less. It requires proof - hard data - that it works the same way in your body.
That’s why generics are safe. That’s why they’re affordable. And that’s why, when your doctor switches you from a brand to a generic, you can trust the change.
The system isn’t perfect. Some complex drugs still take years to approve. Some manufacturers cut corners. But the framework - built on science, not guesswork - keeps patients protected.
What is bioequivalence in simple terms?
Bioequivalence means a generic drug delivers the same amount of medicine into your bloodstream at the same speed as the brand-name version. If two drugs are bioequivalent, they’ll work the same way in your body.
Do all generic drugs need clinical trials?
No. Many generics qualify for a biowaiver - meaning they don’t need human studies. This applies to products like eye drops, ear drops, and topical creams where the drug doesn’t enter the bloodstream. For most oral pills, however, a clinical study in healthy volunteers is required.
Why is the 80-125% range used for bioequivalence?
The 80-125% range is based on decades of data showing that if a generic drug’s absorption falls within this range compared to the brand, patients will have the same therapeutic outcome. It accounts for normal differences between people, like metabolism and digestion. Going outside this range increases the risk of under- or overdosing.
Are generic drugs always as safe as brand-name drugs?
Yes - if they’re approved by the FDA. Every generic must prove bioequivalence and meet the same quality standards as the brand. The FDA inspects manufacturing facilities for both. The only difference is cost. The active ingredient, dosage, and effectiveness are identical.
Why do some generic drugs seem to work differently?
Sometimes, patients notice differences because of inactive ingredients - like fillers or dyes - which can affect how fast a pill dissolves. But if the generic is bioequivalent, the active drug’s absorption is the same. If you feel a change, talk to your doctor. It could be a different generic from a different manufacturer, or it could be unrelated to the drug itself.
How long does it take to get a generic approved?
On average, it takes 14 to 18 months from submission to approval. The biggest delay comes from incomplete or poorly designed bioequivalence studies. Companies that follow the FDA’s product-specific guidances get approved much faster - often 3 months quicker.
Raushan Richardson
December 27, 2025 AT 04:28I used to be super skeptical about generics until my doctor switched me to one for my blood pressure med. Saved me like $80 a month and I haven’t noticed a difference. The FDA’s got this figured out.
Robyn Hays
December 27, 2025 AT 15:21Love how the FDA doesn’t just take ‘trust us’ as an answer. It’s wild to think that a pill’s bioavailability is measured down to the nanogram - like, we’re talking about tiny, invisible differences that could mean the difference between healing and harm. The 80/125 rule isn’t arbitrary; it’s science with skin on. And the fact they’ve tightened it for NTIDs? That’s patient-first thinking right there.
John Barron
December 29, 2025 AT 10:21It is imperative to note that the bioequivalence paradigm, as codified by the FDA in 21 CFR § 320.21 et seq., is not merely a regulatory formality but a pharmacokinetic imperative grounded in the principles of Fick’s law of diffusion and first-order elimination kinetics. The 80–125% confidence interval is derived from logarithmic transformation of AUC and Cmax, and the assumption of normality in log-space - a statistical model validated by over 300 peer-reviewed studies since 1992. To suggest otherwise is to misunderstand the very foundation of clinical pharmacology.
Furthermore, the notion that ‘some generics work differently’ is often a nocebo effect amplified by pharmaceutical marketing. The inactive ingredients are not bioactive; they do not alter pharmacokinetics. If you feel a difference, it is psychological, not pharmacological.
Anna Weitz
December 29, 2025 AT 22:46They say generics are the same but why do I feel weird when I switch? I think they hide stuff in the fillers. You ever read the label? Talc. Corn starch. Dyes. Who knows what’s really in there? They’re cutting corners. And the FDA? They’re too cozy with Big Pharma. This whole bioequivalence thing is just a smokescreen.
Jane Lucas
December 30, 2025 AT 13:36my doc switched me to a generic for my thyroid med and i was nervous af but honestly no change. i even checked my labs and everything was spot on. i think people freak out over nothing. the science is solid.
Miriam Piro
January 1, 2026 AT 12:16Let me tell you something they don’t want you to know… The FDA allows biowaivers for eye drops? That’s because they’re being paid off by the big pharma lobby to let cheap imports slip through the cracks. And don’t get me started on the PBPK modeling - that’s just computer fantasy. Real medicine is tested on humans, not in some Silicon Valley simulation. They’re turning healthcare into a video game. Next thing you know, they’ll approve pills based on TikTok trends. I’ve seen the documents. They’re hiding the real failure rates. 43% first-pass failure? That’s a cover-up. The real number is 70%. They just don’t publish the rest.
Caitlin Foster
January 2, 2026 AT 10:06Wait - so you’re telling me that for $3, I can get the SAME DRUG as the $200 brand? And it’s been tested in 36 people who fasted and had blood drawn like a sci-fi movie? I’m not just buying a generic - I’m buying a superhero origin story. 🦸♀️💊 #GenericWin
Todd Scott
January 3, 2026 AT 13:00For those unfamiliar with global regulatory frameworks, it’s worth noting that the FDA’s 80–125% bioequivalence range aligns closely with the European Medicines Agency’s (EMA) and the World Health Organization’s (WHO) guidelines, demonstrating international scientific consensus. In contrast, some low-income countries still rely on dissolution testing alone, which is insufficient for drugs with variable absorption. The U.S. system, while complex, prioritizes patient safety over speed. The Domestic Generic Drug Manufacturing Pilot Program is a strategic move - reducing reliance on foreign supply chains, especially after the pandemic exposed critical vulnerabilities. This isn’t just pharmacology - it’s public health infrastructure.
Andrew Gurung
January 4, 2026 AT 17:10Oh wow. So we’re just supposed to trust that some lab in India, using a machine that’s probably older than my dad, can replicate a drug made by a Nobel Prize-winning chemist? And you call that ‘science’? I mean, I’ve seen the packaging. The font is different. The logo is smaller. That’s not bioequivalence - that’s a knockoff. You wouldn’t buy a Rolex made in a garage. Why are you swallowing one?
Paula Alencar
January 5, 2026 AT 17:22It is imperative to underscore that the rigorous bioequivalence protocols established by the U.S. Food and Drug Administration represent not merely a regulatory standard, but a moral imperative in public health policy. The 80–125% confidence interval, derived from statistically robust, double-blind, crossover trials, ensures that the therapeutic equivalence of generic pharmaceuticals is not left to conjecture, but is empirically validated. Furthermore, the implementation of Product-Specific Guidance documents - numbering over two thousand and continuously updated - reflects an unprecedented commitment to precision, transparency, and scientific integrity. The fact that these standards are consistently applied to drugs with narrow therapeutic indices, such as warfarin and levothyroxine, demonstrates a profound respect for patient safety. One must recognize that this system, while imperfect, remains among the most scientifically defensible and ethically responsible regulatory frameworks in the history of medicine. To undermine it is to endanger the lives of millions who rely on affordable, effective therapeutics.