Buying a generic drug should be simple - it’s cheaper, just as effective, and approved by the same agencies that oversee brand-name pills. But if you’ve ever looked at a little white tablet and wondered, Is this really the same thing? - you’re not alone. Counterfeit drugs are real, and they’re getting harder to spot. The good news? You don’t need to be a pharmacist to tell the difference. With a few simple checks, you can walk into any pharmacy and know for sure whether your generic medication is legitimate.
What Makes a Generic Drug Legitimate?
A legitimate generic drug isn’t a copycat - it’s a legally approved twin. It contains the exact same active ingredient, in the same strength, and works the same way in your body as the brand-name version. The FDA requires generics to prove they’re bioequivalent: meaning your body absorbs them at the same rate and to the same extent as the original. Studies show 98.7% of approved generics meet this standard, with absorption rates within 1% of the brand-name drug.
What you won’t find in a legitimate generic? Harmful fillers, wrong doses, or fake chemicals. The FDA inspects over 2,500 manufacturing facilities every year. If a plant doesn’t follow strict quality rules (called cGMP), it gets shut down. That’s why legitimate generics from companies like Teva, Sandoz, or Mylan are among the safest drugs you can take.
How Generic Drugs Look Different From Brand Names
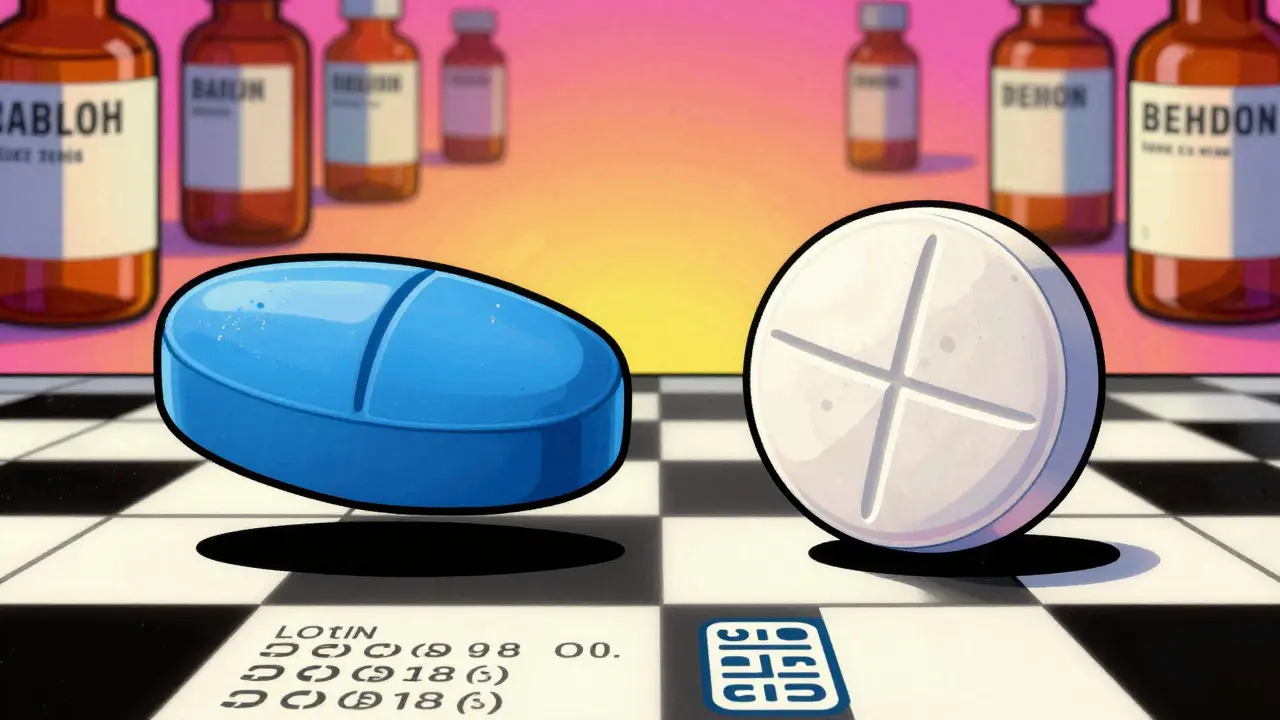
Here’s where people get confused. A generic pill might look nothing like the brand-name version. That’s by design. U.S. law says generic manufacturers can’t copy the exact shape, color, or logo of a brand-name drug - it’s to avoid trademark infringement. So if your brand-name pill is a blue oval and your generic is a white round tablet, that’s normal. It doesn’t mean it’s fake.
What should stay the same? The imprint. Every legitimate pill has a marking - a letter, number, or symbol pressed into it. That’s how pharmacists and regulators track it. If your generic has no imprint, or the imprint is blurry, faded, or missing entirely - that’s a red flag. Legitimate pills are made in factories with precision equipment. They don’t have rough edges, cracks, or uneven coloring.
Check the Packaging Like a Pro
The container tells you more than you think. A legitimate generic comes in a sealed prescription bottle with a clear, professional label. Look for these five things:
- The drug name (like “Lisinopril”) - not just “blood pressure pill”
- The strength (like “10 mg”)
- The manufacturer’s name (Teva, Mylan, Sandoz - not “Pharma Co.” or something vague)
- The lot number and expiration date
- The pharmacy’s name and address - not just a website URL
Counterfeit drugs often have crooked labels, typos, or foreign language text on U.S.-marketed products. If the bottle smells weird - like chemicals or mold - don’t take it. Real medications don’t have odors. And if your pills come in a plastic bag instead of a sealed bottle? That’s almost always illegal.
Verify the Pharmacy Before You Buy
Where you buy matters more than you realize. The National Association of Boards of Pharmacy (NABP) runs a program called .pharmacy - a trusted seal that means the pharmacy meets strict safety standards. You can check if your pharmacy is verified at nabp.pharmacy. If they’re not on the list, walk away.
Online pharmacies are the biggest risk. The FDA says 96% of websites selling drugs without a prescription are fake. If a site lets you buy pills without a prescription, offers prices that seem too good to be true (like $5 for a 30-day supply of Lipitor), or doesn’t have a physical address or phone number - it’s a trap. Even if the pills look right, they could be dangerous.
Stick to local pharmacies you know, or ones with VIPPS accreditation. Pharmacists at these places check every generic against manufacturer databases before handing it over. They know what’s real and what’s not.
Use the FDA’s Tools to Confirm
The FDA keeps a public database called the Orange Book. It lists every approved generic drug and links it to the brand-name version. You can search by drug name and see if your generic is approved and what its manufacturer is. Look up the product code on the label - if it’s not in the Orange Book, it’s not legitimate.
Since 2023, most prescription drugs in the U.S. carry a 2D barcode or serial number. Apps like MediSafe let you scan the code and instantly verify the drug’s origin. Major manufacturers like Teva and Sandoz have already rolled this out on all their U.S. products. If your generic doesn’t have one - ask why.
And if you’re ever unsure? Call the FDA’s MedWatch hotline. Report any suspicious pills - even if you’re not sure. They track patterns. One report might stop a batch of fake pills from reaching hundreds of people.
Red Flags You Can’t Ignore
Here’s a quick checklist of warning signs:
- Tablets that crumble when you touch them
- Pills with inconsistent scoring lines (the lines meant to split the pill)
- Color changes between refills - unless your pharmacist says it’s a new manufacturer
- Missing or mismatched lot numbers
- Expiration dates that are too far in the future (fake pills often have fake dates)
- Unusual taste or aftertaste - especially metallic or bitter
- Pills that don’t work like they used to - sudden loss of effect
One woman in Ohio reported her generic blood pressure pill stopped working after three months. She checked the lot number and found it wasn’t listed in the FDA database. It turned out to be a counterfeit made in China. She ended up in the hospital. Don’t wait for that to happen.
What to Do If You Find a Fake
If you suspect a drug is fake:
- Stop taking it immediately
- Keep the packaging and pills - don’t throw them away
- Call your pharmacist and ask them to verify it
- Report it to the FDA’s MedWatch program at 1-800-FDA-1088 or online at fda.gov/medwatch
The FDA gets thousands of reports every year. Most come from patients who noticed something off. Your report helps protect others.
Why This Matters
Generic drugs save the U.S. healthcare system over $370 billion a year. They’re safe, effective, and essential. But that system only works if people can trust it. Counterfeit drugs don’t just waste money - they can kill. The WHO estimates 1 in 10 medical products in developing countries are fake. While the U.S. has strong protections, the risk is still real - especially with online purchases.
Legitimate generics aren’t a compromise. They’re the standard. You’re not getting less - you’re getting the same medicine at a fair price. But you have to know how to spot the difference.
Next time you pick up a prescription, take 30 seconds. Check the imprint. Read the label. Verify the pharmacy. You’re not being paranoid - you’re being smart.

Roshan Aryal
January 5, 2026 AT 18:08Let me guess - you’re one of those people who thinks the FDA is some kind of infallible deity. Wake up. The same agencies that approve generics also approved the opioid crisis. If you’re trusting a white pill because some bureaucrat stamped it ‘approved,’ you’re already dead inside. Real medicine doesn’t need a label to be real - it needs results. And results? They’re not in the database. They’re in the blood.
Jack Wernet
January 6, 2026 AT 13:21Thank you for this meticulously researched and calmly presented guide. In an era of misinformation, articles like this serve as vital anchors of public trust. The emphasis on verified pharmacies and the Orange Book is particularly commendable - these are not mere suggestions but foundational pillars of pharmaceutical safety. I hope this reaches every community center, senior group, and rural clinic.
Catherine HARDY
January 6, 2026 AT 14:15Did you know the FDA allows generics to be made in China? And that China’s regulatory system is controlled by the Communist Party? What if the ‘Teva’ label is just a sticker? What if the whole supply chain is a front? I once saw a pill with a barcode that scanned to a server in Shenzhen - no FDA inspection records, no U.S. phone number, no traceable owner. They’re not just selling pills - they’re testing bioweapons on Americans. And they’re using your grandma’s blood pressure meds as the delivery system.
Vicki Yuan
January 6, 2026 AT 19:26This is such an important topic! I’ve had patients come in terrified because their generic pill looked different - and honestly, most of them didn’t know about imprints or lot numbers. I always tell them: ‘If it’s from a licensed pharmacy and the imprint matches the FDA’s database, you’re safe.’ I even print out a quick reference sheet for my older patients. Knowledge is power - and it’s free.
melissa cucic
January 7, 2026 AT 23:47It is, indeed, a profound and often overlooked truth - that the very mechanisms designed to ensure public safety - the FDA, the Orange Book, the cGMP protocols - are not merely bureaucratic formalities, but living, breathing safeguards, meticulously maintained through decades of scientific rigor and institutional accountability. To dismiss these systems as arbitrary, or worse, corrupt, is not only intellectually lazy, but dangerously negligent. The fact that 98.7% of generics meet bioequivalence standards is not a statistic - it is a testament to human ingenuity, discipline, and the quiet heroism of regulatory science.
saurabh singh
January 8, 2026 AT 05:11Bro, this is gold. I’ve been buying generics for years from my local pharmacy here in Texas - never had an issue. But man, I never knew about the imprint thing. Now I check every pill like it’s a QR code. And yeah, if it’s not from a real pharmacy? Don’t touch it. I even told my cousin in Delhi to avoid those shady online pharmacies - they’re selling fake insulin there. Stay sharp, fam.
Dee Humprey
January 8, 2026 AT 16:06My mom took a generic for her cholesterol last year and had a weird reaction. I checked the imprint, lot number, and even called the pharmacy - turns out it was legit, just a different manufacturer. She was freaking out until I showed her the FDA page. You’re right - it’s not about the color. It’s about the code. Scan it. Verify it. Breathe. 💊
Allen Ye
January 9, 2026 AT 18:23Consider the philosophical underpinnings of pharmaceutical equivalence: if a molecule is identical in composition, in concentration, in dissolution rate, and in pharmacokinetic profile, then the ontological identity of the substance is preserved - regardless of the shape, the color, the manufacturer’s logo, or the cultural symbolism attached to the brand. Yet society clings to branding as if it were a sacrament, as if the corporate identity of the drug conferred efficacy, when in truth, it is the molecular architecture - the precise arrangement of atoms - that heals. The fear of the generic is not a fear of inferiority - it is a fear of disenchantment. We have been conditioned to believe that price equals worth, and that familiarity equals safety - but science does not care for our illusions.
josh plum
January 11, 2026 AT 14:52Everyone’s talking about the FDA like it’s Jesus with a clipboard. But here’s the truth: the FDA gets paid by the drug companies. They inspect plants for a day - the plant cleans up for the visit, then goes back to cutting corners. And don’t even get me started on the ‘.pharmacy’ seal - it’s just a logo. Anyone can pay for it. I’ve seen fake pills with perfect labels, perfect imprints, perfect barcodes. The only thing that’s real? Your gut. If it doesn’t feel right - it isn’t. And if you’re not checking the manufacturer’s website directly? You’re playing Russian roulette with your liver.
John Ross
January 11, 2026 AT 20:50From a supply chain integrity standpoint, the critical failure point isn’t the pill itself - it’s the serialization layer. The FDA’s DSCSA mandate requires pedigree tracking from manufacturer to dispenser, yet only 42% of community pharmacies have full interoperability with the EPCIS protocol. Without end-to-end traceability, the Orange Book is a historical artifact, not a real-time verification tool. If your generic lacks a 2D Data Matrix code compliant with GS1 standards, you’re not just at risk - you’re operating in a regulatory vacuum.
Clint Moser
January 13, 2026 AT 07:23so i was thinkin… what if the imprints are fake too? like what if the whole system is hacked? i read somewhere that the barcodes can be cloned using a phone camera and a printer. and the lot numbers? they’re just random strings. i scanned mine and it said “approved” but the server was in a data center in the philippines. and the pharmacist? he’s probably paid off. i think the gov is using this to track us. my pills are watching me.
Ashley Viñas
January 14, 2026 AT 05:45How quaint. You assume people care enough to check imprints or lot numbers. Most Americans can’t tell the difference between a pill and a M&M. They swallow whatever’s handed to them - because they’re too lazy, too distracted, too addicted to convenience. And now you want them to ‘verify’ their meds? Please. The real public health crisis isn’t counterfeit drugs - it’s public apathy. And you? You’re just giving them a checklist to feel better about their negligence.
Mandy Kowitz
January 16, 2026 AT 02:08Wow. A whole article about how to check a white pill. Meanwhile, my insurance just raised my copay from $5 to $15 for the same generic. But sure, let’s all become pill detectives while the real scam - the corporate price gouging - keeps going. 🙄